GDP
GOOD DISTRIBUTION PRACTICE
Maintaining product safety and quality during distribution is crucial in the pharmaceutical industry. Drug companies show that they care about suitable marketing methods by ensuring quality. In this regard, Good Distribution Practices (GDP) certification ensures quality. GDP is an excellent way to run retail and distribution sites dealing with medicines. It promises that global rules will be followed and the best standards will be met.
First and foremost, controlling how medicines are stored, treated, and moved is essential. Therefore, suitable marketing methods guarantee the quality of these things. Distributors must ensure that their actions align with the rules. Thus, quality control methods will be the same throughout the supply chain. For example, transferring raw materials to factories is part of the process. It also pays for the shipping of finished drugs to people who will use them. This evaluation checks whether foreign GDP standards are being met and shows that they align with the rules.
Consequently, it also ensures that your method for quality control meets the requirements. An outside evaluation is critical. It thoroughly assesses and confirms that all processes align with global GDP rules. Moreover, this method helps parties trust each other, giving them confidence that standards will be strictly followed. Hence, the safety and effectiveness of medicinal goods are protected. Thus, it is imperative to maintain these practices throughout the marketing process. Lastly, GDP verification is essential. It meets the top standards for quality and safety in the pharmaceutical business.

CERTIFICATION AND AUDITING SERVICES BY CERTPRO
CertPro offers professional help for GDP certification. Therefore, to ensure GDP compliance, we do thorough reviews. Our team promises to follow GDP rules and guidelines. Consequently, we look at how you do marketing and process. In addition, GDP certification shows that you follow global standards, which builds trust among the customer. Hence, trust CertPro to give you expert advice and support. We help you through the complicated process of getting GDP certification.
WHY CHOOSE CERTPRO FOR GDP CERTIFICATION AND AUDITING?
If you need Good Distribution Practice (GDP) certification, you can trust CertPro. Therefore, we also offer audit services. As a result, we have sufficient experience in this field and have served multiple clients for almost ten years. Again, we know how complicated distribution control can be. Why you should pick CertPro: we offer professional advice and quality services at affordable prices.
| Factors | CertPro Advantage |
|---|---|
| Time to Certification | 4x faster than traditional approaches |
| Price | Competitive rates with flexible options |
| Process | Streamlined and efficient methodology |
| Expertise | 10+ years of industry experience |
CERTPRO’S COST-EFFECTIVE APPROACH TO GDP CERTIFICATION
CertPro provides GDP certification services at a low cost. Therefore, we understand the budget concerns regulatory complaints. Consequently, CertPro ensures you will pay for your required services, saving you money. For a smooth certification process, we keep interruptions to a minimum and make the best use of resources. Therefore, CertPro offers GDP compliance strategies and solutions that are both reasonable and effective. Trust CertPro for GDP certification services that will keep the bank intact.
| No. of employees | Timeline | Cost (approx.) |
| 1 – 25 | 4 weeks | 2500 USD |
| 25-100 | 6 weeks | 3500 USD |
| 100-250 | 6-8 weeks | 5000 USD |
| 250 plus | 8 weeks | Custom plans |
UNDERSTANDING THE BASICS OF GDP
The main goals of GDP are safely moving and keeping medicines and active ingredients. Ensuring the standard of production all along the supply line is very important. Small changes in temperature or humidity can affect the quality and efficiency of medicines. Because pharmaceuticals are expensive, they need protection from stealing. GDP certification assesses the lifecycles of healthcare products, particularly prescription drugs for humans. Therefore, it emphasizes safe storage and transportation processes. Compliance audits ensure all distribution chain requirements maintain pharmaceutical quality and integrity.
The European Medicines Agency (EMA) requires GDP to set basic standards for wholesalers. These standards ensure that medicines maintain quality and integrity throughout the supply chain.
Compliance with GDP ensures:
-
Medicines in the supply chain meet EU legislation.
-
Medicines are stored and transported under suitable conditions.
-
Contamination and cross-contamination risks are minimized.
-
Adequate turnover of stored drugs is maintained.
-
Correct products reach recipients promptly and reliably.
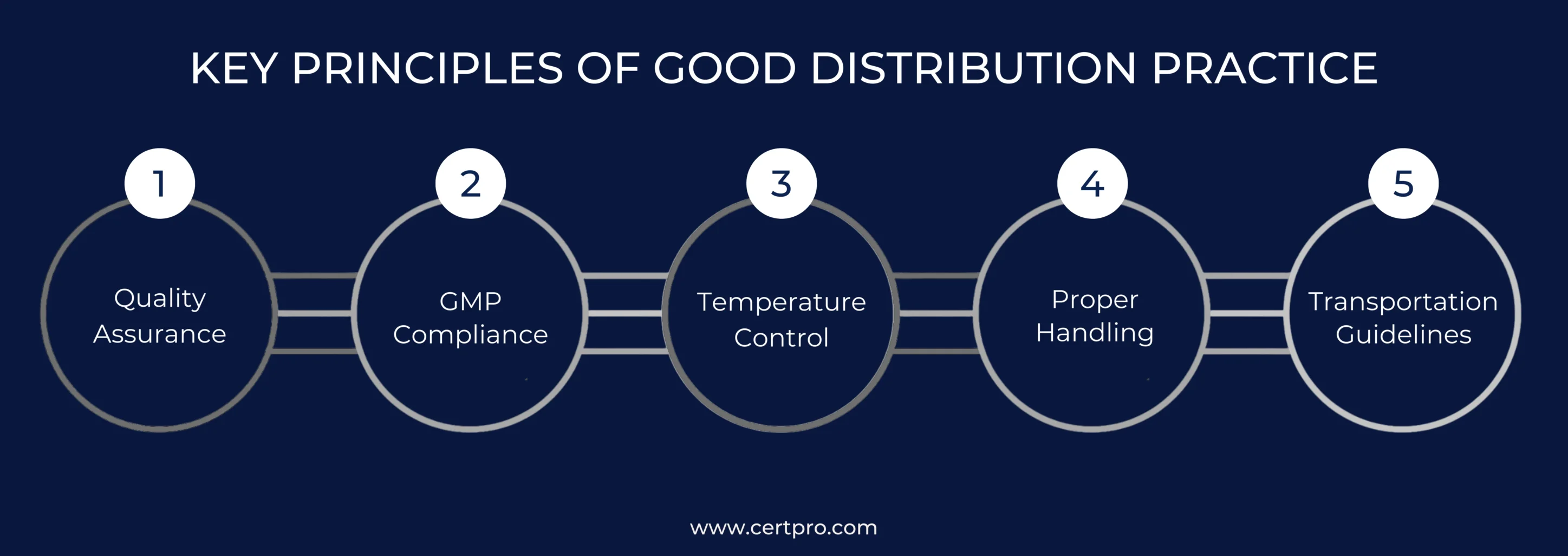
KEY PRINCIPLES OF GOOD DISTRIBUTION PRACTICE
It is imperative to uphold pharmaceutical products’ quality, integrity, and safety during the whole distribution chain. Furthermore, the key principle of GDP certification plays a crucial role. Additionally, the following principles are the foundation of GDP certification:
Quality Assurance: Good distribution practices actively incorporate quality assurance measures. These measures ensure the highest quality of pharmaceutical products. These measures include testing products for potency and purity, conducting stability testing, and maintaining comprehensive product records.
GMP Compliance: Appropriate distribution methods should be used to guarantee adherence to good manufacturing standards (GMPs). Following GMPs is crucial in the pharmaceutical industry. It ensures that the products are safe and effective.
Temperature Control: Following proper distribution standards should include managing the temperature of pharmaceutical products. Temperature control is essential to preserving the quality and efficacy of medications during storage and transportation.
Proper Handling: Good distribution processes should guarantee proper handling of pharmaceutical items. This avoids medication harm and verifies that they are kept at the appropriate temperature.
Transportation Guidelines: Good distribution practices should include guidelines for the safe and secure transportation of pharmaceutical products. These guidelines may encompass aspects such as using suitable packaging. Additionally, they ensure proper labeling for transport.
THE CERTIFICATION PROCESS FOR GDP
Organizations must follow reasonable distribution procedures to get GDP certification. This ensures customers receive high-quality products. Here are the steps on how to get a GDP certificate:
Initial Consultation and Goal Definition: Identify your company’s needs and goals during this phase. Define your organization’s objectives for GDP certification and create a customized plan that fits your unique requirements.
Pre-audit and Project Planning for Audit: These are crucial steps to prepare for the audit. Project planning meetings coordinate audits for sites or departments. The pre-audit phase identifies vulnerabilities and takes proactive measures before the final audit.
Stage 1 and Stage 2 Audits: During the certification check, auditors examine your controls and processes. The audit focuses on cold storage buildings, sensor technology, and maps. Monitoring, process safety, weather records, and evacuation plans are also necessary. Auditors also examine security steps in managing and handling the products. Finally, the auditor suggests recommendations for improvement.
System Evaluation: After the GDP audit, an independent certification board examines the data. If the group meets all the conditions, the board grants GDP compliance. This step ensures that the company is serious about using a suitable marketing strategy.
Surveillance Audits and Recertification: The process is rechecked yearly, helping to determine what needs to be fixed. Recertification also takes place before the GDP certificate expires. This ensures that cooperation continues and shows that the organization is serious about maintaining high standards.
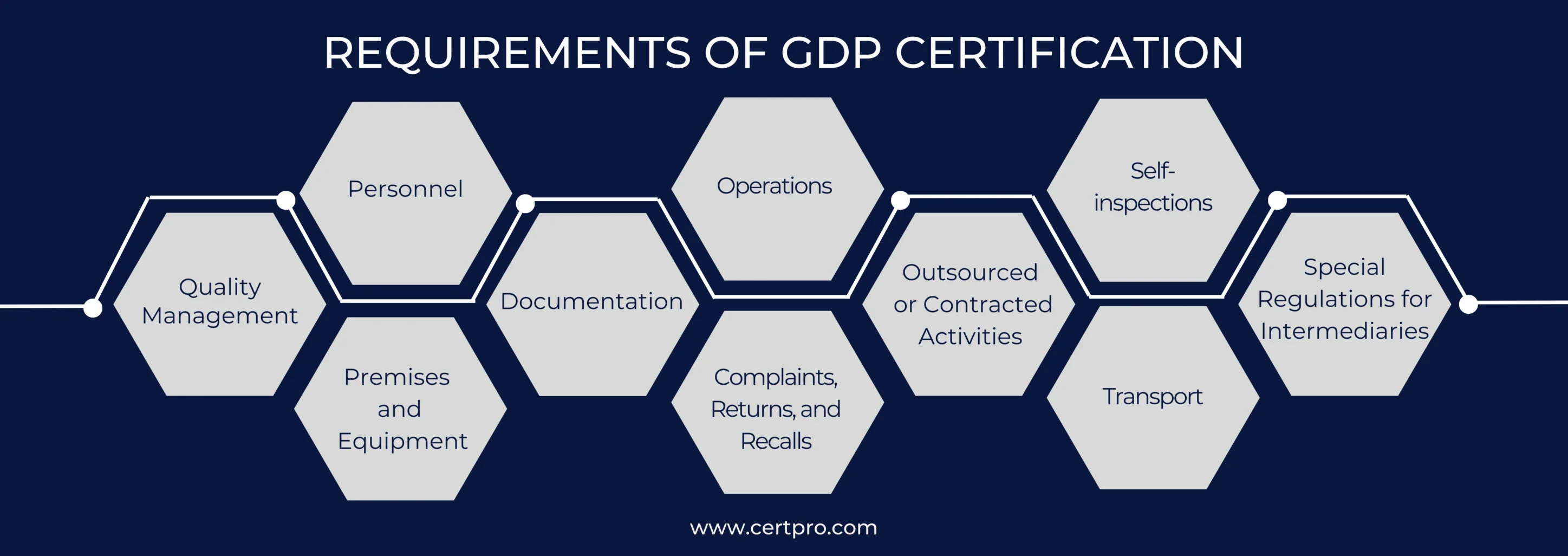
REQUIREMENTS OF GDP CERTIFICATION
The EU’s Good Distribution Practice Guidelines inform people how to handle medicines properly. These rules ensure that the goods keep their quality and purity and that different parts of the marketing process follow specific rules.
Quality Management: GDP certification requires a quality system that meets standards. This includes organizational structure, procedures, resources, and actions. A documented quality policy and authorized procurement and release procedures are essential. Regular reviews and revisions based on risk assessments ensure continuous improvement.
Personnel: A responsible person must be appointed to maintain the quality system and oversee the GDP training program. The training covers product security and the identification of fake medicines. The distributor keeps records of all GDP training and conducts regular assessments.
Premises and Equipment: The premises and equipment requirements for GDP include:
- Secure and sound storage areas with enough capacity
- Adequate lighting and prevention of unauthorized access
- Segregated areas for different pharmaceutical products
- Proper design and maintenance for cleanliness
- Regular cleaning and pest control
- Weather protection for receiving and dispatching bays
- Contamination prevention during handling and storage
- “First expiry/first out” (FEFO) principle for products with expiration dates
- Separation of broken or damaged items
- Dedicated areas with safety measures for hazardous or sensitive products
Documentation: GDP documentation includes clear titles and purposes. It requires approval by an authorized person and adherence to retention periods set by authorities.
Operations: GDP operations require proper handling, storage, and transportation of pharmaceutical products. Accurate records, quality risk management, and product security measures are essential.
Complaints, Returns, and Recalls: Complaints are categorized as quality-related or distribution-related. The wholesaler reports quality complaints to the manufacturer and investigates distribution complaints thoroughly. Returns are assessed based on product nature and storage conditions, excluding stolen products. Recalls involve notifying the manufacturer, regulatory authorities, and customers. Progress is documented in a final report.
Outsourced or Contracted Activities: Contract activities in GDP involve authorized parties with written consent. Contracts define responsibilities, adherence to GDP principles, and measures to prevent counterfeit products. All contract acceptors must comply with GDP requirements. Subcontracting is allowed with written approval. Regular audits of contract acceptors are required.
Self-Inspections: Self-inspections are part of the GDP quality system. They monitor compliance, identify areas for improvement, and facilitate corrective measures. Conducted by a competent person, they result in thorough records and suggested actions. Management ensures effective follow-up and evaluation of reports.
Transport: Wholesalers verify the validity of delivery orders or have a replenishment plan. Medicines are labeled and placed in containers with handling instructions. Wholesalers protect medicines from damage, contamination, and theft during transit. Vehicle operators report any deviations to the wholesaler and recipient promptly.
Special Regulations for Intermediaries: Entities involved in pharmaceutical distribution must ensure compliance with good distribution practices. This maintains product integrity and safety throughout the supply chain.
BENEFITS OF GOOD DISTRIBUTION PRACTICES
GDP accreditation benefits the pharmaceutical sector greatly and includes good distribution techniques. Among these advantages are:
Ensuring the Quality of Pharmaceutical Products: Good distribution practices ensure the quality of pharmaceutical products. They regulate their handling, movement, and storage. These regulations play a significant role in the pharmaceutical industry. They safeguard patients by ensuring they have access to adequate and safe treatments.
Maintaining GDP Certification for Quality Assurance: Complying with best distribution practices is necessary to keep GDP certification active. Businesses can guarantee customers constant product quality by upholding compliance. This pledge satisfies the highest quality requirements.
Empowering Companies Through Training: GDP training is essential as it gives businesses information on how to distribute goods well. In addition, this training makes it possible to follow the rules set by regulators. It gives businesses the tools they need to become and stay GDP-compliant. In this way, they make sure that business norms are always followed.
Protecting Patients: GDP protects patients from poor distribution practices. It ensures medications are appropriately stored, transported, and handled, preserving their quality and effectiveness. These guidelines safeguard patients and guarantee safe and effective medications.
Enhancing Product Quality: GDP enhances product quality by regulating storage, transportation, and handling. Proper temperature maintenance and damage prevention are crucial; adequate handling preserves medication quality.
Achieving Higher Sales Volumes: Good distribution practices boost sales volumes. Customers trust companies that provide high-quality medications, which leads to increased purchases and higher sales volumes. GDP enhances product quality by regulating storage, transportation, and handling. Proper temperature maintenance and damage prevention are crucial; proper handling preserves medication quality.
ELIGIBILITY FOR GDP CERTIFICATION
Many businesses in the pharmaceutical supply chain can get GDP certification. Pharmaceutical makers, dealers, wholesalers, and companies also help with logistics. GDP certification can also be given to companies that store medicines. In addition, people who deliver and carry things are also skilled. The approval has nothing to do with how big the company is. Also, where you live has nothing to do with your status.
Moreover, any sizeable distributor or global logistics firm can apply for GDP accreditation. However, they must fulfill the prerequisite requirements and abide by the authorities’ requirements. Hence, obtaining GDP accreditation demonstrates the organization’s dedication and guarantees the integrity and quality of pharmaceutical items.
THE COST OF GDP CERTIFICATION
Several variables can affect GDP certification costs. However, these include the organization’s size, complexity, certification body, and process scope. Charges usually cover application fees, assessment fees, and paperwork reviews. Additionally, they include on-site audits and ongoing surveillance. However, extra costs may arise. As a result, These are for upgrades or fixes. Such needs might appear during certification.
Moreover, the costs of GDP certification are justified by the necessity. Therefore, it ensures that high-quality standards are maintained. In addition, it promises that the products are safe and meet the standards of the law. Organizations should look at the long-term effects of GDP. Some of these perks are a better image, more customer trust, and more market competition. This way, they can determine whether getting and keeping certification is worth the money.
VALIDITY OF GDP CERTIFICATION
The certification for Good Distribution Practices (GDP) has continued for five years. It begins with the date of the most recent audit and can only be renewed or reissued with further audit. Therefore, organizations must undergo new inspections to ensure the certification’s validity and conduct necessary follow-up procedures. Thus, the surveillance audit process helps to maintain GDP standards and evaluates the organization’s adherence to guidelines.
Furthermore, companies should take proactive measures by scheduling inspections. They should also carry out follow-up activities. This helps maintain the validity of their GDP certification. By doing so, they demonstrate their commitment to good distribution practices. Consequently, they ensure ongoing compliance and integrity in their operations.
CERTPRO’S SUPPORT FOR GOOD DISTRIBUTION PRACTICE CERTIFICATION
CertPro is an expert in assisting companies in obtaining GDP certification. Consequently, they also provide thorough consultation, auditing, and certification services. CertPro helps your company get certified by providing a team of knowledgeable auditors. Therefore, we guarantee adherence to industry norms and optimal procedures. We also evaluate your distribution methods and point out areas that need improvement. CertPro aids in the deployment of efficient QMSs. In addition, we also offer professional advice and assistance with documentation. As a result, it increases client trust and meets regulatory standards. As a result, obtaining GDP certification with CertPro’s support also enhances your credibility. It improves operational efficiency and generates new commercial prospects in the pharmaceutical industry.
FAQ’s
WHAT IS THE SIGNIFICANCE OF GDP CERTIFICATION?
GDP certification is crucial for maintaining the quality and safety of pharmaceutical products throughout the distribution process. It helps protect patients, assures customers of product quality, and ensures compliance with regulatory requirements.
WHAT HAPPENS IF AN ORGANIZATION FAILS TO MEET GDP REQUIREMENTS?
Failure to meet GDP requirements can result in several consequences for an organization, including non-compliance penalties, reputational damage, legal ramifications, and potential risks to patient safety. It is crucial for organizations to prioritize and consistently adhere to GDP guidelines to uphold the quality and integrity of pharmaceutical products throughout the distribution process.
HOW LONG DOES IT TAKE TO OBTAIN GDP CERTIFICATION?
The time required for GDP certification depends on various factors, including the organization’s readiness, the complexity of operations, and the certification body’s process. Typically, the certification procedure takes many months to complete.
ARE THERE ANY SPECIFIC REQUIREMENTS FOR POST-CERTIFICATION ACTIVITIES?
Post-certification, the company consistently complies with GDP guidelines, promptly takes any necessary corrective action, and actively participates in certification body surveillance audits. These activities ensure that the organization sustains its adherence to quality standards and demonstrates its commitment to upholding the principles of good distribution practices.
IS GDP CERTIFICATION MANDATORY?
GDP certification is not universally mandatory, but it is often required or strongly recommended by regulatory authorities and industry stakeholders. It demonstrates a commitment to good distribution practices, ensuring the quality, integrity, and safety of pharmaceutical products. Many organizations voluntarily seek GDP certification to enhance their reputation and meet customer expectations.
NAVIGATING DATA PRIVACY FRAMEWORKS: A COMPREHENSIVE GUIDE
Globalization has intense effects on business functioning and scaling. In today's digital world, companies are generating an unprecedented rate of data that requires protection from emerging cyber threats. In addition, recurring data breaches and privacy concerns make...
BUSINESS NON-COMPLIANCE: THE HIDDEN FINANCIAL AND OPERATIONAL COSTS
Businesses are always in a dilemma regarding whether or not to be compliant. Most companies think that compliance will problematize their operating process. However, highly regulated industries like financial and healthcare services meet the legal obligations for...
Security Frameworks: A Comprehensive Guide with 14 Examples
Technological advancements make cyberattacks more sophisticated and advanced. Hence, organizations must keep up with the latest cybersecurity frameworks in these complicated scenarios to sustain themselves in a dynamic threat environment. Different cybersecurity...