ISO 17025:2017
TESTING AND CALIBRATION LABORATORIES
The ISO 17025 certification certifies that the labs have complied with regulations. Thus, it demonstrates proficiency, objectivity, and consistency in calibration and testing. It is essential for labs of all sizes. The value of certification ensures the availability of resources and dependable procedures for precise outcomes. It increases credibility, standing, and client confidence. The certification process helps to get accurate results in forensic sciences, food processing, and medicines.
Furthermore, accreditation has many advantages. It lowers errors, boosts productivity, and raises the caliber of testing. Global markets recognize accredited labs, and their findings gain credibility. Obtaining certification facilitates adherence to rules and promotes continuous development.

Certification and Auditing Services by CertPro
At CertPro, we understand quality management and the benefits of ISO 17025:2017 certification. Thus, we offer extensive support to organizations in achieving the certification. Our skilled experts will help you through the process and ensure your lab meets ISO requirements. Moreover, we collaborate closely with your team and develop a tailored QMS. This system meets your specific needs and aligns with industry standards. We can help you set up this system correctly so your lab can quickly get approval for ISO 17025:2017.
Why choose CertPro for ISO 17025:2017 certification and auditing?
CertPro is a reliable partner for ISO 17025:2017. Additionally, we offer top-notch auditing services. Consequently, with nearly ten years of experience, we understand testing and calibration well. Furthermore, we grasp the complexities involved. Here are key reasons to choose CertPro. We have extensive expertise. In addition, through our knowledge and experience, we help our clients a great deal. We think CertPro is the best choice for your ISO 17025:2017 certification needs because of the following:
| Factors | CertPro Advantage |
|---|---|
| Time to Certification | 4x faster than traditional approaches |
| Price | Competitive rates with flexible options |
| Process | Streamlined and efficient methodology |
| Expertise | 10+ years of industry experience |
CertPro’s Cost-Effective Approach to ISO 17025:2017 Certification
CertPro offers reasonably priced services for ISO 17025:2017 certification. We prioritize cost reduction and compliance, which are crucial for companies. Therefore, our customized strategy ensures you only pay for necessary services, saving money. Although we minimize interruptions and optimize resources for a smooth certification process. CertPro provides practical strategies and affordable solutions for effectively achieving HIPAA compliance. Therefore, businesses can rely on CertPro for a cost-effective path to ISO 17025:2017 certification.
| No. of employees | Timeline | Cost (approx.) |
| 1 – 25 | 6 weeks | 4750 USD |
| 25-100 | 8 weeks | 6750 USD |
| 100-250 | 8-10 weeks | 9750 USD |
| 250 plus | 12 weeks | Custom plans |
UNDERSTANDING ISO 17025:2017 STANDARD
ISO 17025 certification is essential, as it sets standards for lab proficiency and ensures the quality of services. This standard ensures that lab results are correct and reliable. By following a set of rules, labs can show that they are skilled in technology and unbiased, which makes people trust them. ISO 17025 can be used in different types of labs, such as public, private, university, and research labs.
The standard encompasses several vital aspects. First, it sets out the requirements for management systems. Second, it ensures the lab’s technical competence. Third, it addresses the need for proper equipment and measurement traceability. It also covers handling test items and specifies how to report results accurately. Laboratories can deliver reliable and trustworthy results by following ISO 17025. It proves their competence and enhances their credibility.
Additionally, they comply with international practices, which builds customer confidence. Regulatory bodies trust certified labs and accreditation authorities recognize their reliability. The standard promotes quality management systems and encourages risk assessment. In addition, it supports continuous improvement and ensures valid and reliable processes. Overall, ISO 17025 is crucial for labs, providing accuracy and fostering trust in testing.
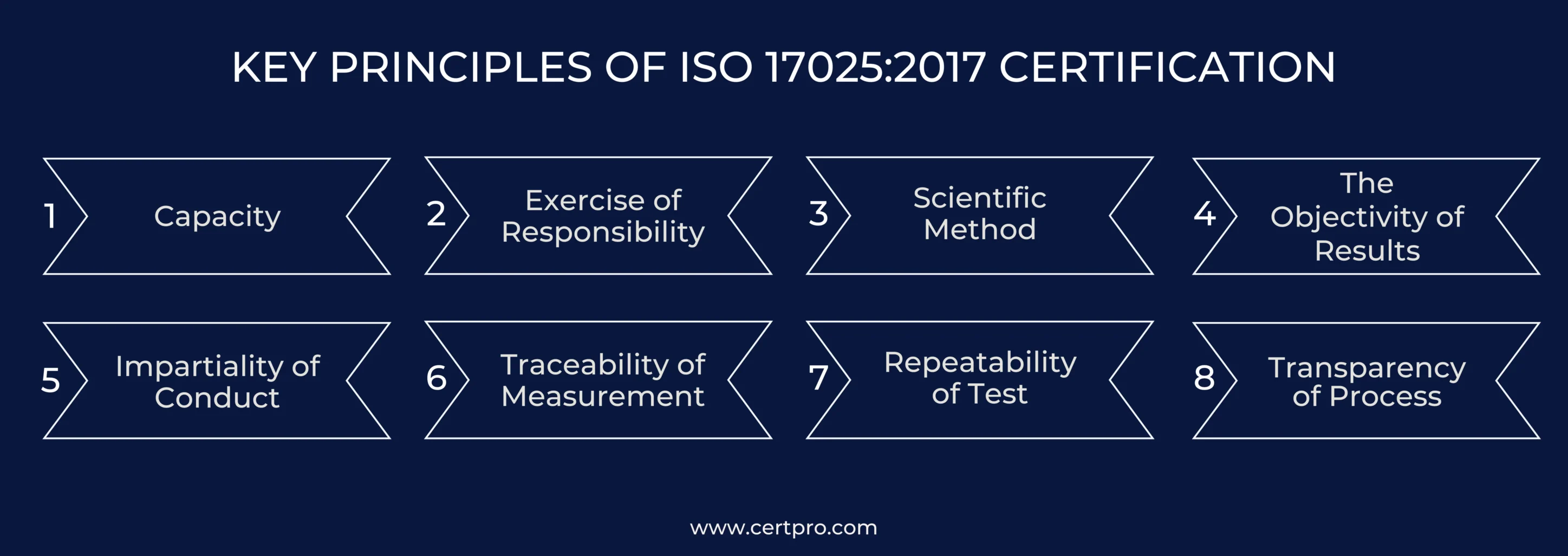
KEY PRINCIPLES OF ISO 17025:2017 CERTIFICATION
The principles underlying ISO 17025 certification are crucial.
Capacity: Laboratories must have the right resources. It must have skilled staff, proper facilities, and equipment. They also need quality control measures and established procedures. These resources help labs perform well and ensure valid and reliable results.
Exercise of responsibility: Responsibility is key. Labs must allocate authority, define specific roles, and ensure accountability for outcomes. Responsibilities should be clear and executed well. Integrity is vital in all tasks.
Scientific Method: The scientific method is essential. Labs should follow accepted scientific approaches. However, these methods should be agreed upon in the field. Any deviations must be justified. Adhering to strict scientific practices is essential for maintaining credibility and reliability.
The Objectivity of Results: Objectivity in results is crucial. Results should be based on measurable quantities. Subjective results are an exception and should be handled only by qualified individuals. These results must be identified as subjective. Experts in the field should be aware of this distinction.
Impartiality of Conduct: Impartiality is essential to ensuring unbiased and accurate results. Testers must prioritize valid results and adhere to accepted scientific methods. Other influences should be secondary and must not take precedence over scientific integrity.
Traceability of Measurement: The traceability of measurements is crucial for ensuring accuracy and reliability in laboratory results. Labs must maintain a transparent comparison chain linking their devices to recognized standards. This process guarantees that measurements are consistent and trustworthy. An assessment of measurement uncertainty is included to validate the results further.
Repeatability of Test: Another critical principle is the repeatability of tests. Tests should yield consistent results, which require the same procedures and equipment. The same personnel should conduct tests, and results should remain consistent over time.
Transparency of Process: Transparency of the process is also essential. Laboratory operations must be thoroughly examined through internal and external assessments. These evaluations help find problems and ensure the results are correct and scientifically sound.
These principles cover many requirements. Consequently, they provide a broad understanding for lab staff and guide assessors. Although professional judgment is essential in evaluations, adhering to these principles ensures reliability.
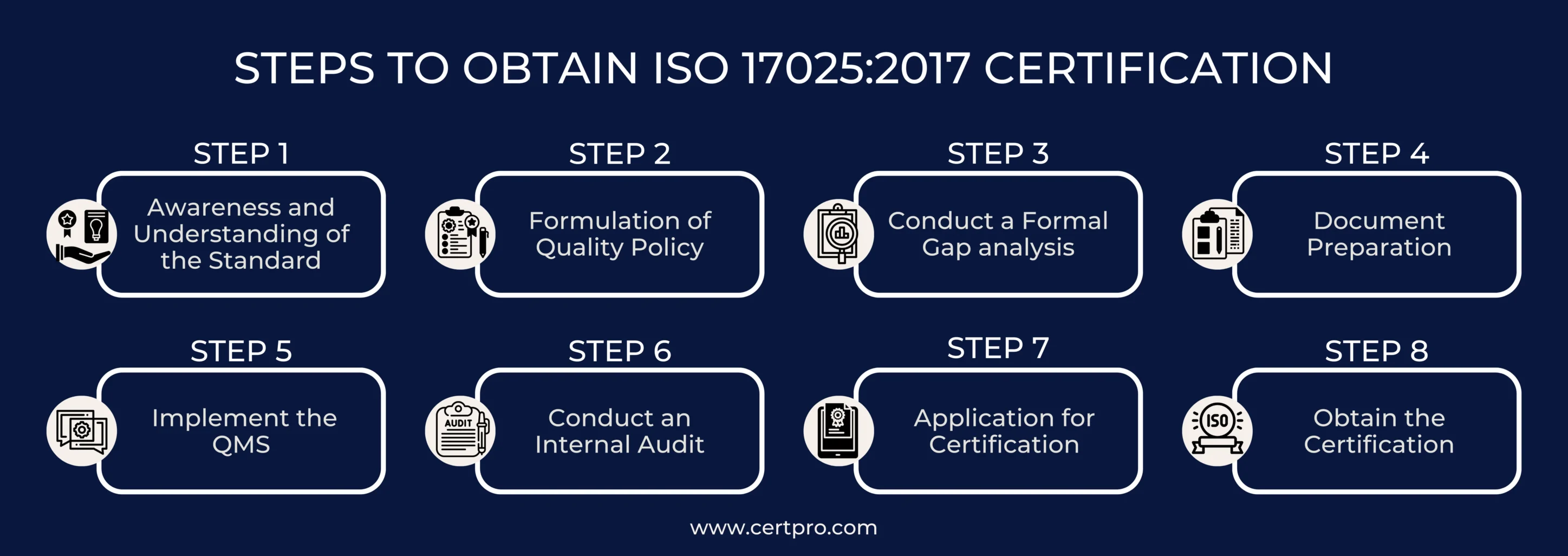
STEPS TO OBTAIN ISO 17025:2017 CERTIFICATION
ISO 17025 certification is essential for labs to show competence in testing and calibration. Labs must follow steps to become certified, which ensures compliance with global quality standards and earns recognition for reliability.
Step 1: Awareness and Understanding of the Standard: First, learn ISO 17025 terms. Then, train staff for QMS implementation. Additionally, ensure that everyone knows the new lab practices.
Step 2: Formulation of Quality Policy: Create a brief policy for your lab’s QMS and ensure it complies with ISO 17025 standards. Include goals such as management reviews, staff participation, and continuous improvement. Use the SMART model for setting goals: specific, measurable, achievable, relevant, and time-bound. Customize the policy to fit your lab’s unique circumstances. This ensures it is suitable and workable for your operations.
Step 3: Conduct a Formal Gap Analysis: Conduct a thorough gap analysis to assess your lab’s operations. This ensures compliance with ISO standards for Quality Management Systems (QMS). Identify areas of non-compliance to gauge your current compliance level accurately. This analysis will pinpoint areas where improvements are needed. By addressing these gaps, you can work towards achieving full compliance.
Step 4: Document Preparation: Prepare your QMS and lab operations documentation. First, have them checked by external auditors. Next, ensure all documents are accurate and up-to-date. Those important documents include the quality manual, procedures, formats, test procedures, work instructions, and validation methods.
Step 5: Implement the QMS: Successfully implement the Quality Management System (QMS). Assign staff members duties and obtain from management the resources that they require. Create a thorough checklist to help guarantee that the QMS requirements are followed. This strategy will sustain high compliance rates and enable smooth implementation.
Step 6: Conduct an Internal Audit: Conduct a thorough internal audit to see how effectively your actions align with the Quality Management System. Therefore, appoint skilled auditors who understand the ISO standard. The audit will reveal any non-conformities in your organization. You will receive a report outlining the necessary adjustments and corrective measures. This method is critical for addressing non-conformities.
Step 7: Application for Certification: Search for registered certification bodies and choose one that aligns with your preferences. Apply and receive a price quote and an estimated timeframe for the certification assessment. If the terms are acceptable, proceed with the certification process.
Step 8: Obtain the Certification: The chosen certifying authority will conduct a comprehensive on-site evaluation. Your Quality Management System (QMS) and operations are assessed as part of this assessment. The certifying authority will examine records and evaluate the compliance status. They will also watch testing methods in action and conduct staff interviews. Additionally, they will check the calibration apparatus. If no problems are found during the examination, the certification authority will confirm that ISO standards are being followed. Finally, they will award certification following a successful verification.
In addition, they will notify you after identifying non-conformities or discrepancies. Therefore, you must rectify them within a specified timeframe to obtain the certification.
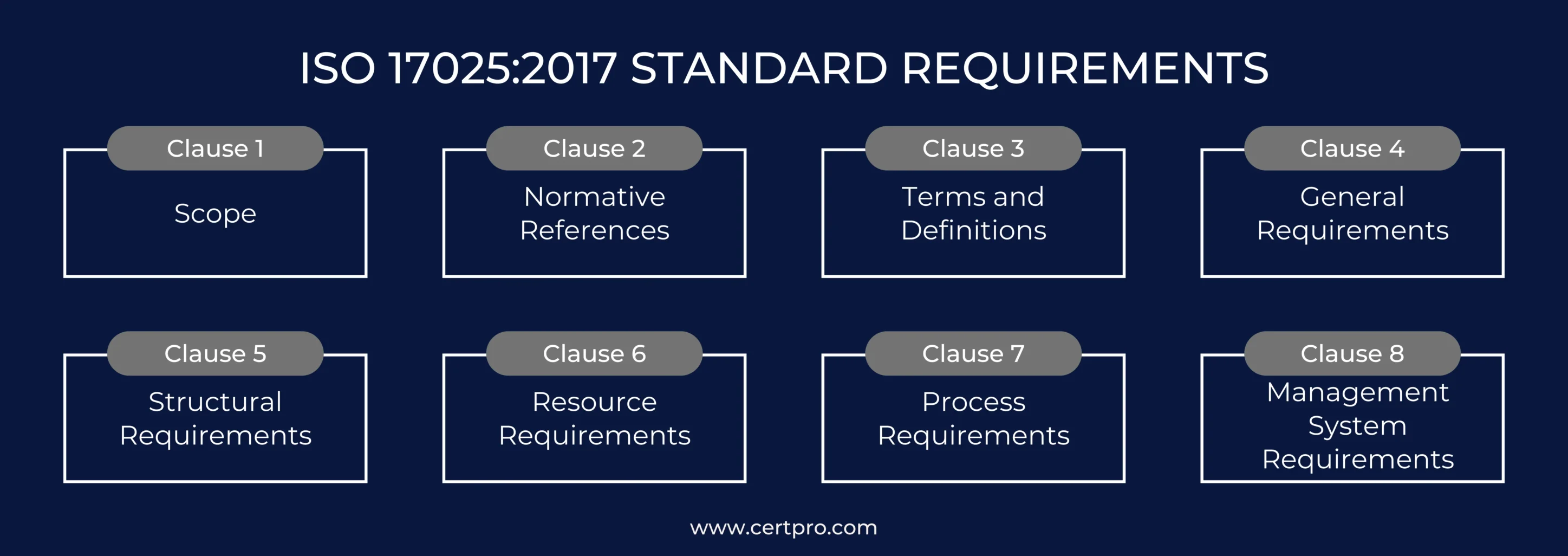
ISO 17025:2017 STANDARD REQUIREMENTS
Organizations must comprehend several of the standards to comply with ISO 17025 requirements. The 2017 edition of ISO 17025 contains significant modifications. It improves management requirements and provides new general and structural requirements in clauses 6 to 8. This includes resources, processes, and management systems. This edition makes general recommendations to help labs verify their competence and impartiality.
The ISO 17025 standard consists of the following clauses and elements:
Clause 1. Scope: Discuss the objective of the standard, its applicability, and the purpose of ISO 17025.
Clause 2. Normative References: Provide details on how specific guides and standards are referenced within the requirements to ensure consistent implementation and compliance.
Clause 3. Terms and Definitions: Defines the terminology used throughout the standards.
Clause 4: General Requirements: It addresses two primary requirements: impartiality and confidentiality. This ensures labs maintain integrity and safeguard sensitive information.
Clause 5: Structural Requirements: This clause covers structural requirements, including organizational structure, components, and processes, which ensure effective and organized lab operations.
Clause 6: Resource Requirements: It outlines the essential components required by a laboratory. This clause covers general requirements, including facilities and environmental conditions. It also involves personnel, metrological traceability, equipment, and externally provided products and services.
Clause 7: Process Requirements: The 11 key processes enhance efficiency and meet standards. These include handling requests, contracts, methods, validation, sampling, and records. Consequently, these processes ensure consistent, reliable, and accurate lab operations.
Clause 8: Management System Requirements: It highlights the two options for organizations to comply with the standard.
Option A applies to laboratories that maintain a separate Quality Management System (QMS). This system is dedicated solely to their operations.
Option B pertains to laboratories integrated within larger organizations or those that have established management systems already aligned with ISO 9001:2015 standards.
The clause addresses essential management system tasks, including tasks that require addressing risks and opportunities. It also streamlines procedures and conducts corrective actions. These criteria ensure that laboratories’ management systems are trustworthy. Thus, supporting ISO 17025 standards, these solutions encourage consistent testing and calibration procedures.
BENEFITS OF ISO 17025:2017 CERTIFICATION
By achieving ISO 17025 accreditation, your laboratory gains several benefits:
- Reputation Boost: The widely used ISO 17025 standard makes lab testing and calibration more believable and reliable.
- Compliance Assurance: The certificate ensures safety rules are followed, legal responsibilities are met, and customers are happy.
- Performance Guideline: ISO 17025 encourages regular competency and data quality, ensuring correct and reliable findings.
- Increased Business: The approval increases customer trust, improves the brand’s image, and gives it an edge in global opportunities.
- Time and Cost Savings: It improves operational effectiveness, decreases supplier audits, and eliminates retesting.
- Global Business Connections: ISO 17025 promotes new alliances and cooperation by facilitating trade internationally.
- Reduced Customer Complaints: Following ISO 17025 guidelines reduces errors, which raises customer satisfaction.
- Competitive Edge: Accredited labs have a significant advantage over rivals.
- Technology Development and Usage: ISO 17025 encourages the adoption of new technologies for improved testing procedures.
Proper Equipment Maintenance: The standard promotes maintenance and calibration for accurate and reliable results.
THE COST OF ISO 17025:2017 CERTIFICATION
The cost of ISO 17025 certification might vary significantly for various reasons. These considerations include the laboratory’s location, size, accreditation scope, and infrastructure. Laboratories should be prepared to invest significantly, possibly hundreds of thousands of dollars. However, sharing costs with other sections or programs can reduce expenses. Initially, costs include purchasing software and equipment like LIMS and document control systems. Annual maintenance fees are generally affordable.
Furthermore, location affects the running cost, including salaries for quality assurance and auditor travel expenses. In addition, existing policies, procedures, equipment, and software may influence certification costs. Laboratories with a robust quality management system may see lower expenses.
CERTPRO’S ASSISTANCE IN ACHIEVING ISO 17025:2017 CERTIFICATION FOR YOUR BUSINESS
CertPro offers comprehensive auditing, consulting, and certification services. As a result, we support your firm in acquiring ISO 17025 certification. Therefore, our professional personnel guarantees adherence to industry standards. Additionally, we assess management systems for conformance and use quality controls. Hence, we offer extensive documentation and guidance.
Furthermore, we provide training to improve testing and calibration accuracy. Thus, CertPro allows you to get ISO 17025 certification quickly. Consequently, choosing CertPro reflects a dedication to quality and continual development in laboratory services. Similarly, we enable a seamless certification procedure and increase trust in laboratory operations.
FAQ’s
HOW LONG DOES IT TAKE TO GET ISO 17025 CERTIFIED?
The timeframe for ISO 17025 certification depends on the laboratory’s readiness and the certification body’s process. It typically takes several months to a year to complete the certification process.
WHAT ARE THE CHANGE IN ISO 17025:2017?
ISO 17025:2017 introduced several changes, such as replacing Management requirements and Technical Requirements with General Requirements and Structural Requirements. It also added clauses for Resource Requirements and Process Requirements, placing greater emphasis on laboratory competence and impartiality.
WHAT IS THE CONSEQUENCE OF ISO 17025 NON-COMPLIANCE?
Non-compliance with ISO 17025, the international standard for testing and calibration laboratories, can result in various consequences that impact the reputation, operations, and legal standing of a laboratory.
WHAT IS THE SIGNIFICANCE OF ISO 17025 ACCREDITATION FOR A LABORATORY?
ISO 17025 accreditation enhances a laboratory’s reputation, ensures compliance with quality standards, promotes reliable testing and calibration, increases customer confidence, and opens doors to international business opportunities. It also helps in improving operational efficiency and reducing errors.
CAN ISO 17025 CERTIFICATION BE REVOKED?
Yes, ISO 17025 certification can be revoked if a laboratory fails to maintain compliance with the standard or address identified non-conformities. Regular surveillance audits and re-certification assessments are conducted to ensure continued adherence to the requirements.
NAVIGATING DATA PRIVACY FRAMEWORKS: A COMPREHENSIVE GUIDE
Globalization has intense effects on business functioning and scaling. In today's digital world, companies are generating an unprecedented rate of data that requires protection from emerging cyber threats. In addition, recurring data breaches and privacy concerns make...
BUSINESS NON-COMPLIANCE: THE HIDDEN FINANCIAL AND OPERATIONAL COSTS
Businesses are always in a dilemma regarding whether or not to be compliant. Most companies think that compliance will problematize their operating process. However, highly regulated industries like financial and healthcare services meet the legal obligations for...
Security Frameworks: A Comprehensive Guide with 14 Examples
Technological advancements make cyberattacks more sophisticated and advanced. Hence, organizations must keep up with the latest cybersecurity frameworks in these complicated scenarios to sustain themselves in a dynamic threat environment. Different cybersecurity...